[September 2022] Biden’s Biomanufacturing Initiative and Cancer Moonshot Needs Key Investment to Hit the Mark
Data show U.S. Offshore Biomanufacturing Policy may lead to failure without investment in innovation and training
The success of the President’s Biomanufacturing Initiative announced on September 14th and the Cancer Moonshot will need more than an ‘America First’ manufacturing strategy, according to data from BioPlan’s 19th Annual Report of Biopharmaceutical Manufacturing Capacity and Production. Over the past two decades, the U.S. has retained the lead position as the world’s outsourcing destination to produce biologics, such as cancer therapeutics and vaccines. Creating incentives for more domestic U.S. production capacity will not change this position.
The Problem: To maintain the U.S.’s continued dominance in biopharmaceutical manufacturing will require an investment of up to $500 million to fix the nation’s inability to hire skilled manufacturing staff. This represents half of the $1 billion the White House announced on September 14th that the U.S. DoD will invest over five years to ‘catalyze the establishment of the domestic bioindustrial manufacturing base.’
For more than 10 years, U.S. biologics manufacturers have complained explicitly that they cannot find the workers needed to keep production in-house. Indeed, over the past 16 years, since 2006, the percentage of biologics manufacturers producing everything in-house has declined from 57.6% to 34.9%. The fact that nearly two-thirds of the biologics manufacturers are outsourcing is a clear indication that something systemic is broken.
- COVID-19 has accelerated this migration, creating even more demand for manufacturing alternatives to in-house production. In our study this year, we found that nearly eight of 10 manufacturers expect to be outsourcing at least some of their production by 2026.
- The two major centers for biopharmaceutical manufacturing are the U.S., with 27.8% of global capacity and Europe, with 30.8%. China, with around 13.2% of global capacity, includes a significant percentage that is built but is not yet being fully utilized (see BioPlan’s www.top1000bio.com database).
The U.S. will likely remain the number-one global biologics manufacturing country in terms of overall capacity and production, by a large margin. However, over the past 20 years, the rest of the world has been quietly developing its own domestic capacity. China’s growth, in particular, over the past 10 years has shown expansion from low single-digits to the number two capacity-holder. Indeed, in China, WuXi Biologics took the number two spot for global contract production revenue this year.
A Solution: More production expertise is needed across the board. The innovations in emerging areas like cell and gene therapies have begun to pull expertise, staff, and resources from mainstream biologics production. On top of the steadily increasing global demand for skilled biomanufacturing expertise, this has created a big staffing gap. We found 51.3% of companies developing advanced therapies cannot find and hire the production staff needed. If a company cannot commercialize its products because it cannot manufacture them, they will consider alternatives such as offshoring to someone who may have the staff and expertise. Decision-makers will certainly consider offshoring if the available expertise happens to be outside the U.S.
This is a clear cause-and-effect: Lack of staff with expertise leads to outsourcing manufacturing to wherever capabilities exist. The industry’s highly effective Pandemic response has clearly shown us that physical capacity is not holding back the industry. What is needed is a deeper bench of production expertise. This is vital to maintaining the U.S.’s competitiveness and is a critical part of the equation for the Cancer Moonshot.
Fixing this systemic lack of skilled local manufacturing expertise will require more than just adding a few STEM programs in middle schools. Workforce investment has been talked about for decades, and many excellent training programs exist in the U.S., Europe, and elsewhere. But this is not enough. The nagging hiring problems are not going away. The data show we are simply not keeping up with the demand for process development, operations, and engineering.
If the President’s initiatives result in greater physical manufacturing capacity in the U.S. without addressing systemic staffing problems, we will risk mothballing facilities with idle capacity because there will not be enough qualified expertise to run them.
Creating an experienced staffing base is a long-term investment, but the U.S. can create a competitive advantage in manufacturing by preparing engineers and process scientists that will position the U.S. well for the future. However, the president’s announcement to stimulate domestic biomanufacturing to support his Cancer Moonshot ignores a market reality: The U.S. is, in fact, already the preferred destination for global outsourcing. (see Fig 1).
Fig 1: Destination for Global Outsourcing
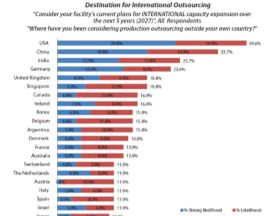
“The President’s Cancer Moonshot needs to challenge existing thinking that manufacturing is about physical capacity,” said Eric Langer, managing partner at BioPlan Associates. “The full weight of the Federal Government could ignite the thinking required to deliver the education and training results necessary to create the manufacturing side of the Moonshot the President expects.”
Retaining Domestic Manufacturing is Not Enough
Global biologics innovators already see the U.S. as the preferred destination for manufacturing their biologics. BioPlan’s 19th Annual Report and Survey of Biopharmaceutical Manufacturing Production and Capacity shows how the industry has changed over the past two decades. For example, cell and gene therapies can be at the center of the President’s Cancer Moonshot. But these will require manufacturing systems quite different from what has been used for decades in biopharmaceutical manufacture.
COVID has accelerated the industry’s ability to manufacture vaccines more efficiently with less and those lessons are already being applied to other manufacturing. But refocusing efforts on next-generation medicines, including cell and gene therapies, antibody-drug conjugates, and other potentially disruptive clinical technologies will drive the Cancer Moonshot.
Further, because healthcare issues like cancer know no borders, when the U.S. creates innovative cures and then has the expertise to produce these biologics with trained and skilled staff, this will create healthcare solutions that will ensure the U.S.’s current strong competitive advantage in biomanufacturing for the long-term.
Others will not have the expertise due to insufficient skills in quality manufacturing in a regulated environment. In fact, 67.2% of the global industry feel regions such as China, India, and Brazil may be unable to meet international quality standards for biologics. But unless something is done, that advantage may slip away. The ability to offer exceptionally high-quality standards is what sets biologics manufacturing in the US apart. Meeting global standards requires a highly trained staff with sophisticated knowledge. The availability of staff has historically determined whether outsourcing to a destination can even occur, because without that demonstrated expertise, biomanufacturing will fail.
The President’s plan to ignite domestic biomanufacturing is exciting, but the right emphasis needs to be placed on investing in the sophisticated training and education required to expand the U.S.’s global industry position and to ensure its manufacturing competence continues to lead the world.
Eric Langer, managing director of BioPlan Associates, is available to discuss the biomanufacturing elements of the President’s plan. He is available for interviews at +1 240-401-3503, elanger@bioplanassociates.com
1 Research Court, Suite 450
Rockville, MD 20850 USA
+1 301 921 5979
www.bioplanassociates.com
2019 FDA Biopharmaceutical Approvals [PDF] (January 2020)
Advances in Biopharmaceutical Technology in China, 2nd ed [PDF] (October 2018)
15th Annual Report and Survey on Manufacturing [PDF] (September 2018)
13th Annual Report and Survey on Manufacturing – 2016 Trends [PDF] [July 2016]
12th Annual Biomanufacturing Just Released–Industry Growth Continues in 2015 [PDF] (June 2015)
Biopharmaceutical Expression Systems [PDF] (November 2008)
Quick Guide to Clinical Trials (May 2008)
Part 1 Part 2
5th Annual Report Biopharmaceutical Manufacturing [PDF] (April 2008)
Expression Systems Set To Dictate Future of Biopharmaceutical Industry (December 3, 2008)
New Book Demystifies the World of Clinical Trials [PDF] (April 24, 2008)
Advances Biopharmaceutical Technology in China [PDF] (2007)
Biopharmaceutical Manufacturing Capacity to Increase 48% by 2010 According to New Report [PDF] October 2005
Capacity Utilization in Biopharmaceutical Manufacturing Down 8% [PDF] October 2005
34% of Biopharmaceutical Manufacturers Experiencing Capacity Constraints in 2005 [PDF] October 2005
47 Recently Approved Biopharmaceutical Products Included in Biotherapeutics Reference [PDF] February 2004
Improved BioManufacturing Efficiencies Signal Reduced International Healthcare Costs According to New ASM Report [PDF] February 2004



